Work
When a thermodynamic system does work, it uses energy in the form of heat added to the system. The general definition of work is that of a force times distance.
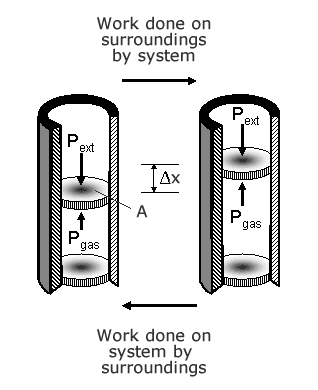
Consider a freely moving piston in a cyclinder which is closed at one end. Inside the cyclinder, there is a volume of gas inside the cylinder which is at a certain temperature T, pressure P and takes up a volume V. If heat is added to the system, the temperature is raised, the molecules in the gas gain kinetic energy and hit the walls of the cylinder with increased velocity. The force on the walls of the cylinder increase. The cylinder walls cannot move, but the piston, which is free to move, experiences a reaction force F=P A, where P is the pressure and A is the area of the piston. The piston moves a distance Δx. The work done dW=∫P A Δx. If A is constant, A Δx is the change in volume. For a constant force, W= PdV.Therefore the work done is P dV.
When the volume of a gas increases, work is done by the gas.
When the volume of a gas decreases, work is done on the gas by an external force.
Quasi-Static Process
If we consider the system to change slowly over time, we can effectively, consider each state to be considered reversible
The work is dependent on the path over which the process takes place.
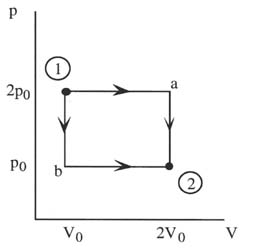
The work done in going from 1 to 2 via a is
W= 2p0V0
but it is
W= p0V0
In going from 1 to 2 via b.
The above result shows that if the temperature of a gas is increased at constant volume, no work is done.
However, if the temperature is increased and the gas is allowed to expand, work will be done. In this case, extra energy will have to be supplied to do this work.
For this reason, gases are said to have two principal specific (or molar) heat capacities:
i) the specific (or molar) heat capacity at constant volume, cv ii) the specific (or molar) heat capacity at constant pressure, cp It should be clear that cp > cv and that the difference between them is given by
cp - cv = pDV
