Electrical Cells and Batteries
Introduction
In it's most simple form a battery can be regarded as a pump that provides the energy to move charge around a circuit.
In order to provide a potential difference, or electro-motive force (EMF) a store of energy is required. One such method is a battery or cell. The common usages of the term battery is any device that converts chemical energy into electrical energy. However, strictly speaking, the term battery is used when several electrical cells are connected together to provide a source of a potential difference in a circuit. If it is just a single chemical source then it is called a cell.
History
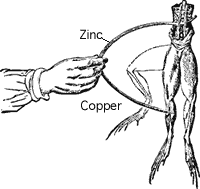
In 1791, Galvani noticed that a circuit created with two different metals, when touched on the ends of the leg of a dead frog, would cause it twitch. The two metals were creating an electric current within the frog's leg, causing the muscles to contract. Early batteries were an improvement of this method transfering chemical energy into electrical energy.
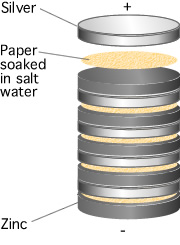
The first battery was invented in 1793 by Alessandro Volta. Just as the two different metals touching the wet skin of a frog's leg, caused an electrical current to flow, early batteries increased the voltage that could be produced by stacking a pile of discs made from silver and zinc sandwiched between paper soaked in a salt water solution as shown in Figure 2. In honour of Volta, we use the Volt as the unit of potential difference and EMF.
Battery Operation
Why does this produce electricity? The flow of current can be understood as the flow of ions from the more reactice metal to the less reactive metal. The ions moving from one electrode to the other creates an electrical charge which is neutralised by the flow of electrons across the wire.
Before considering the reaction of two metals, consider what happens when we place a single metal electrode in an electrolyte. Some of the metal atoms in the electrolyte go into solution as ions while the remaining electrons create a negative charge on the metal. The separation of ions and electrons leads to a separation of charge. However, this build up of charge cannot continue indefinitely because as the negative charge builds up in the metal it becomes increasingly difficult for positive metal ions to go into solution. A similar build up in positive charge in the electrolyte also prevents the build up of charge. This degree of charge build up depends on the metal and represents the work required to separate electrons from the ions. This is known as the electroneutrality principle
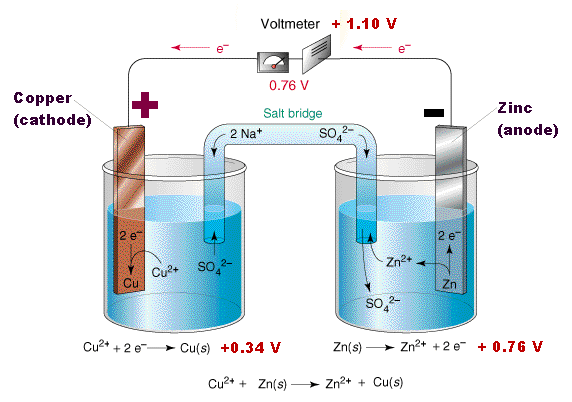
Similarly, if a copper strip is placed in an aquaous Copper(II)Sulfate solution the copper will also lose ions. These reactions are often written as Cu | Cu+2 this is the half-cell reaction.
The tendancy for Zinc to lose ions is greater than that of Copper. When the two cells are joined together (using a copper wire to connect the electrodes and porous barrier that allows the ions to pass known as a salt-bridge connect the elecrolytes, the build up of electrons on the zinc will flow to through the wire onto the copper.
The copper ions in the electrolyte gain electrons and become copper atoms.
Thus the reaction can be written,
Zn | Zn2+ | | Cu2+ | Cu
To continue the reaction, the charge must be removed. This can be acheived by coupling a second reaction which uses the electrons in the metal to convert the ions in the electrolyte into a metal. For a more specific example, consider a zinc electrode in an electrolyte of Copper(II)Sulphate solution.
The loss of electrons by the Zinc is known as oxidation. Zn(s) → Zn2+ + 2e-.(1)
A wire connecting the Zinc electrode to a Copper electrode, allows the electrons to flow to the Copper electrode. Copper ions in the copper sulphate solution take up the electrons and become atoms of copper on the copper electrode. The gaining of electrons by ions is known as reduction
Cu+2 + 2e- → Cu(s).(2)
The net reactions is then,
Zn(s) + Cu2+ → Cu(s) + Zn2+
When the two electrodes are joined by a wire the charge stored can flow and the electrons combine. The simplest kinds of battery have two conductors made of different materials which are partially emersed in a solution which allow the electrons and ions to flow freely known as an electrolyte.
At the copper electrode (cathode), the acid dissolves the copper metal producing hydrogen gas, H+. The reaction will continue until the supply of zinc is used up. The electrons, with their negative charge, are attracted to the copper electrode which causes a current to flow. One of the problems with this cell is that the current stops flowing after a short time because the hydrogen bubles block the current.
Cells using aqueous (containing water) electrolytes are limited in voltage to less than 2 Volts because the oxygen and hydrogen in water dissociate in the presence of voltages above this voltage. Lithium batteries (see below) which use non-aqueous electrolytes do not have this problem and are available in voltages between 2.7 and 3.7 Volts. However the use of non-aqueous electrolytes results in those cells having a relatively high internal impedance.
Activity Series
In the activity series, a metal will give up electrons to any other metal which is below it on the activity series. Elements which gain electrons are called negative irons. Elements which lose electrons are called positive ions. Any more active metal will give up electrons to a less active metal. This will serve to protect the less active metal from corrosion. For example, steel ships often have bars of zinc attached to the sides of the ship. As the steel is corroded by the oxygen of water and air, the zinc will give up electrons to the steel and protect it from corrosion.
Batteries in Circuits
Just like any other electrical component, individual cells can be placed in series or parallel. In series their voltage sum to create a battery with a higher voltage but the current remain the same as in a single cell. In parallel, the batteries have the same voltage but the current is summed to create a battery with a higher current.
Batteries and Internal Resistance
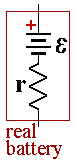
A real battery has internal resistance, r, which lowers the voltage when the cell is connect to a load. If you try to send too much current through a battery, the internal resistance will convert the battery’s own chemical potential energy into thermal energy. The battery will get warm and electrons will leave the negative electrode with relatively little energy.
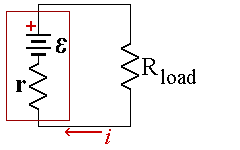
The EMF of the battery is given by E and represents the voltage when the battery is open circuit. If the battery had no internal resistance, the current flowing in the circuit would be given by Ohm's law, I =E/R where R is the resistance of the load.
The internal resistance adds to the total resistance of the circuit. If we call the new total resistance of the cell and load, Req = r + R
Then current is then I= E/Req = E/(r + R).(3)
Since Req is larger than R the current flowing in the circuit is reduced. Therefore, if we are making a cell we want the internal resistance to be a small as possible.
What is the voltage across the resistor R? Clearly it is not the same as the EMF because of the internal resistance. We know that the EMF, E = Ir + IR. We also know that the voltage across the resistor R is IR. Rearranging these two equations we find the voltage in terms of E, r and R.
V = E - Ir (4).
This equation also shows that if we draw a lot of current from the circuit we see the voltage reduces. A good example of this is a car starting with its lights switched on. When the ignitioin key is turned, the electric starter-motor uses a lot of current before it starts to turn. The current for this is supplied by the car battery, which also powers the lights. The voltage available for the lights is reduced at this moment and the lights dim as the car starts. They quickly become bright again once the engine is turning over because less current is required by the starter motor. (When the car has started no current is drawn by the starter motor because it disengages from the engine.)
| Metal | Metal Ion | Reactivity |
|---|---|---|
| Lithium | Li+ | Most Reactive |
| Potassium | K+ | |
| Calcium | Ca2+ | |
| Sodium | K+ | |
| Magnesium | Mg2+ | |
| Aluminum | Al3+ | |
| Manganese | Mn2+ | |
| Zinc | Zn2+ | |
| Chromium | Cr2+, Cr3+ | |
| Iron | Fe2+, Fe3+ | |
| Lead | Pb2+ | |
| Copper | Cu2+ | |
| Mercury | Hg2+ | |
| Silver | Ag2+ | |
| Gold | Au+,Au3+ | Least Reactive |
| Platinum | Pt2+ |
Power and Efficiency
The power output of the battery is given by PE = I2R(5)
But from equation (3), I = E/Req = E/(r + R), therefore, P = E2R/(r + R)2(6)
Similarly, the power given off as heat by the battery to due to internal resistance is Pr = I2r = E2r/(r+R)2(7)
The efficiency, η of the circuit is the ratio of the power actually produced by the battery with its internal resistance to the power supplied by the source, P0. P0 = I E
The power generated is given by I VR, where VR is the voltage across the resistor R.
η = PE/P0 = IVR/IE = R/(r + R)
The smaller the internal resistance, the closer the efficiency, η will be to its maximum value of 1.
If we plot the power tranferred to a load resistance R against the increasing R along with the efficiency we find that the maximum power is transferred by the battery when R = r. This is a very important result and find applications in many electrical devices is known as Jacobi's theorem
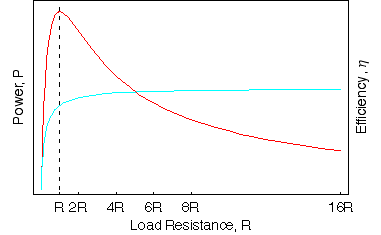
To check this is true we can also differentiate the expression for the power P against R and set it equal to zero to find the maximum value of R.
dP/dR = -dE2/dR (r + R)-2 + d(R+r)-2/dR (E2R) = 0
E2/(r + R)2 - 2 E2R/(r + R)3 = 0
(r + R)3 - 2R(r + R)2 = 0
(r + R) - 2R = 0
r - R = 0 or r = R
While the power transferred may be at a maximum, the efficiency at this percent is only 50%. The higher the load resistance, the greater the efficiency. In practise the exact loading of the circuit is dependent on the application, a good voltmeter has an extremely high-resistance so that the power transmitted is as small as possible.
Types of Battery
Over the years, progress in battery technology has been rather slow but the need for small more powerful batteries in the many small electrical items we carry around with us has driven research into higher power, longer lasting batteries.
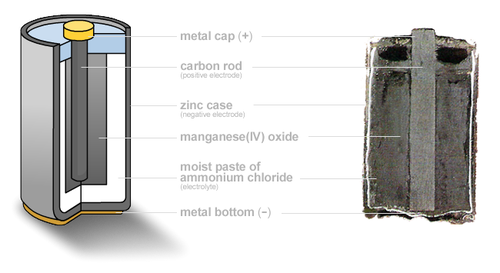
Zinc Carbon
This is commonly known as the Leclanché Cell and despite being the oldest type of battery it is still the most commonly used as it is very low-cost. Traditional Zinc Carbon batteries cannot be reused when their chemical energy has been released
Alkaline Cells
Alkaline chemistry is used in common Duracell and Energizer batteries, the electrodes are zinc and manganese-oxide, with an alkaline electrolyte. Alkaline batteries can be re-used upto 100 times with the correct type of battery charger. A normal battery charger must not be used to charge these batteries.
The active materials used are the same as in the Leclanché cell – zinc and manganese dioxide. However the electrolyte is potassium hydroxide, which is very conductive, resulting in low internal impedance for the cell. This time the zinc anode does not form the container; it is in the form of a powder instead, giving a large surface area.
Silver Zinc
Lightweight but expensive. Used in aeronautical applications.
Rechargable or Secondary Cells
Rechargeable batteries are rechargeable because the chemical reaction that leads to the flow of current is reversible by passing a current through the battery. The animation shows a battery undergoing charging and discharging. When the battery is charged the current can flow through a resistive load.
Lead-Acid
Lead acide batteries are used to provide large amounts of current for a relatively short time. They consist of plates of lead and lead oxide in a solution of sulphuric acid. Lead combines with SO4 (sulphate) to create PbSO4 (Lead Sulphate), plus one electron. Lead dioxide, hydrogen ions and SO4 ions, plus electrons from the lead plate, create PbSO4 and water on the lead dioxide plate. As the battery discharges, both plates build up PbSO4 and water builds up in the acid. The characteristic voltage is about 2 volts per cell, so by combining six cells you get a 12-volt battery.
| half-reaction V | vs SHE |
| Pb + SO42- → PbSO4 + 2e- | .356 |
| PbO2 + SO42- + 4H+ + 2e- → PbSO4 + 2H2O | 1.685 |
Nickle Cadmium (Ni-Cad)
Nickle-Cadmium cells are the most common type of re-chargable battery. They have a high-energy density and a EMF of 1.2 V. They can be recharged more times than other types of rechargable batteries but unless they are fully discharged before recharging suffer from a memory effect which reduces their capacity to store charge.
Nickle-Metal Hydride (Ni-Mh)
Lithium Ion (Li-Ion)
Lithium is the most electronegative metal in the electrochemical series. It also has a low density so it is an atractive material for the anode of batteries. However, lithium is also very reactive with aqueous electrolytes producing hydrogen gas. Because of this it took many years to develop a stable electrolyte. Lithium batteries must be sealed from moisture and air due to the reactivity of lithium. Non-rechargable lithium batteries have been available since the 1980s with rechargable lithium batteries becoming widely available around 1995. Lithium-ion batteries can be recharged between 500 - 1000 cycles. The half-reactions are:
The following reactions take place upon discharge:
Anode: xLi+ + Mn2O4 → LixMn2O4
Cathode: LixC6 → xLi+ + 6C + xe-
Overall: LixMn2O4 + 6C → LixC6 + Mn2O4
Lithium Polymer (Li-poly)
Lithium polymer batteries use a solid polymer electrolyte.
Rusting
Corrosion of iron and steel due to rusting is responsible for millions of pounds of damage each year. Rustiing, is oxidation of the metal to form a metal oxide. Rust does not firmly adhere to the surface of the metal allowing it oxide further. The oxide causes damage to the surface of the metal known as pitting which, over time, reduces thes structural integrity of the metal.
What has this got to do with batteries? Rusting is a chemical process the occurs when the iron or steel is exposed to moist air, it reacts with the oxygen in the air to create Iron (III) oxide. We saw earlier how electricity is generated by the process of oxidization and reduction. The formation of rust can occur at some distance away from theactual pitting or erosion of iron as illustrated below. This is possible because the electrons produced via the initial oxidation of iron can be conducted through the metal and the iron ions can diffuse through the water layer to another point on the metal surface where oxygen is available. This process results in an electrochemical cell in which iron serves as the anode, oxygen gas as the cathode, and the aqueous solution of ions serving as a "salt bridge" as shown below.
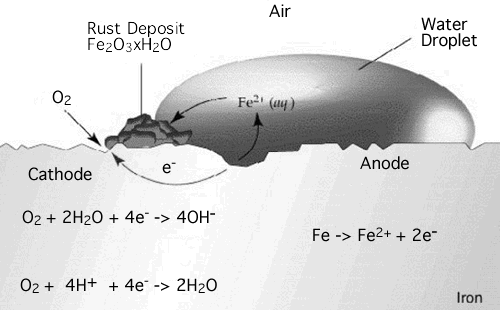
Fe → Fe+2 + 2e- and Fe → Fe+3 + e- in the anode
The amount of water complexed with the iron (III) oxide (ferric oxide) varies as indicated by the letter "X". The amount of water present also determines the color of rust, which may vary from black to yellow to orange brown. The formation of rust is a very complex process which is thought to begin with the oxidation of iron to ferrous (iron "+2") ions.
Fe → Fe+2 + 2 e-
Both water and oxygen are required for the next sequence of reactions. The iron (+2) ions are further oxidized to form ferric ions (iron "+3") ions.
Fe+2 → Fe+3 + 1 e-
Tthe electrons provided from both oxidation steps are used toreduce oxygen as shown.
O2 (g) + 2 H2O + 4e- → 4 OH-
The ferric ions then combine with oxygen to form ferric oxide [iron (III) oxide] which is then hydrated with varying amounts of water. The overall equation for the rust formation may be written as:
Other metals, such as Aluminium, form an oxide layer when they come into contact with oxygen from the air but the layer of oxide bonds very strongly to the surface of the Aluminium preventing further oxidation from occurring. However, Aluminium can rust in a very short time if a thin layer of mercury is applied to the surface. Mercury readily combines with aluminium to form a mercury-aluminum amalgam when the two pure metals come into contact. When the amalgam is exposed to air, the aluminium oxidizes, leaving behind mercury. The oxide flakes away, exposing more mercury amalgam, which repeats the process thus a small amount of mercury can rust a large amount of aluminium over time, by progressively forming amalgam and relinquishing the aluminium as oxide. For this reason mercury is prohibited on aircraft.
Fuel Cells
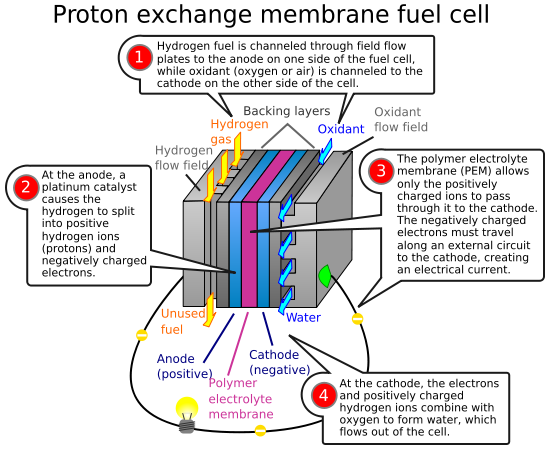
Hydrogen fuel cells are a relatively new technology that take hydrogen and oxgen and convert them into electricity by using a catalyst. Water is the result of the reaction. Thus the hydrogen fuel cell is potentially an environmentally method of powering cars.
Nuclear Batteries
Nuclear batteries may seem like a recipe for disaster given the concern for nuclear safety, however, they have the ability to produce power for long periods of time. Nuclear batteries are not new. It may surprise you to know that they have been been implanted into paitents that suffer from heart arrthymia to power cardiac pacemakers since the 1973. Advances in the power of lithium batteries led to the phasing out of nuclear batteries by 1975. The development of nuclear batteries has been re-ignited with the need for long lasting batteries to power the portable devices such as laptops, mp3 players and mobile telephones.
Nuclear batteries work by converting the heat produced by a nuclear source and creating a current using the Seebeck-effect a second type of nuclear cell uses beta-radiation impinging on a semiconductor junction to create electron hole pair which migrate to the elelectrode of the junction creating a current. Much in the same way that a solar cell creates energy. Currently these batteries cannot produce enough power to run a laptop however they can be used to trickle charge batteries to give longer lifetime for existing batteries.
